Diagnostic labs & pathology
Pulse allows you to edit the checklists from the library to suit your specific needs. With our smart checklist builder and editor, you can infuse logic in your questions for more clarity and better visibility.
SIGN UP TO EDIT TEMPLATES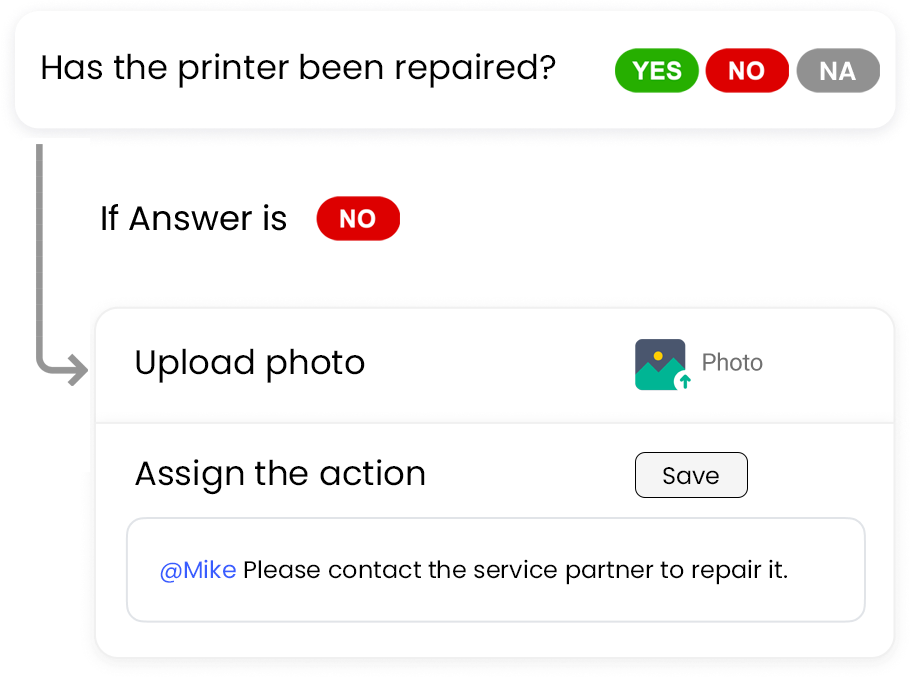
Pulse helps you convert your existing inspection templates into digital ones. Simply upload them in PDF, Excel and Word format and leave the rest for our support team. We will get back to you with digital forms.